World’s First Medical Device Regulatory Approval of Digital Therapeutic App for Hypertension
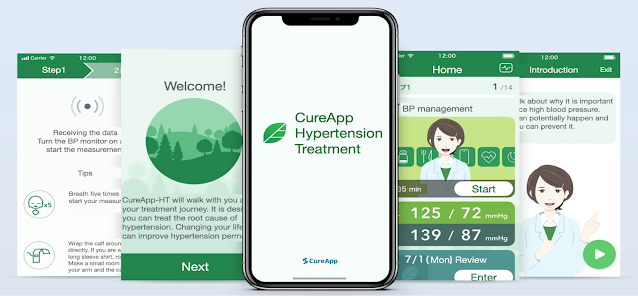
World’s First Medical Device Regulatory Approval of Digital Therapeutic App for Hypertension Mental and Behavioral Approaches to Lifestyle Modification CureApp, Inc. (Head Office: Chuo-ku, Tokyo; CEO: Kohta Satake) was notified by the Ministry of Health, Labour and Welfare on April 26, 2022 that the company had received the medical device regulatory approval of an digital therapeutic app for Hypertension. This marks the first time a standalone software app supporting doctors and patients has received medical device regulatory approval in Japan, and is the first app addressing hypertension to be approved in the world. Preparations are now underway to receive reimbursement and launch the app in 2022. Hypertension Lifestyle modification is a vitally important part of treating essential hypertension, regardless of blood pressure classification. However, lifestyle modifications are dependent on the patient’s mindset and motivation, and workplace and home environment, and are difficult