CureApp and Teijin Pharma Sign Agreement for Joint Marketing and Sales of Prescription Digital Therapeutic App for Hypertension

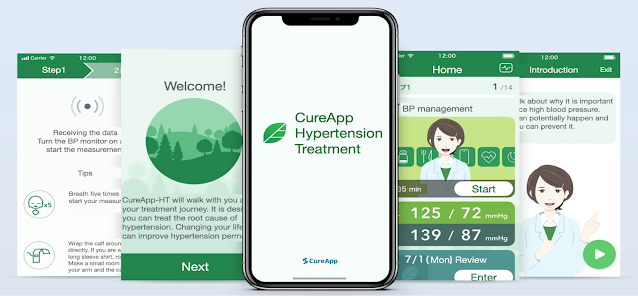
Toward Dissemination of Digital Therapeutics CureApp and Teijin Pharma Sign Agreement for Joint Marketing and Sales of Prescription Digital Therapeutic App for Hypertension Teijin Pharma Limited (Headquarters: Chiyoda-ku, Tokyo, President: Masaki Taneda, hereinafter Teijin Pharma) and CureApp, Inc. ( Headquarters: Chuo-ku, Tokyo, President: Kohta Satake, hereinafter CureApp) have concluded a joint marketing and sales agreement (hereinafter, joint agreement) for the "Prescription Digital Therapeutic App for Hypertension" (hereinafter, CureApp HT) as of April 1st. Through this partnership, we aim to expand the use of CureApp HT, which supports the lifestyle modifications that are a prerequisite to any type of hypertension treatment. Further, through the distribution of digital therapeutics (DTx) *1 , the two companies aspire to address unmet medical needs. *1 Digital therapeutics: medical devices that have been approved by regulatory authorities for their therapeutic effects,...